


Statistica Sinica 13(2003), 539-554
STATISTICAL TESTS FOR POPULATION BIOEQUIVALENCE
Shein-Chung Chow, Jun Shao and Hansheng Wang
StatPlus, Inc., University of Wisconsin and Peking University
Abstract:
In its 2001 guidance, the U.S. Food and Drug Administration (FDA) recommends
that population bioequivalence (PBE) and individual bioequivalence (IBE) be
assessed to address respectively the prescribability and switchability
between a brand-name drug product and its new formulation or generic copy.
For IBE, the FDA recommends a 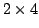 crossover design and a statistical
test procedure proposed by Hyslop, Hsuan and Holder (2000). The same method is
also recommended in FDA (2001) for assessment of PBE under the
crossover design and a statistical
test procedure proposed by Hyslop, Hsuan and Holder (2000). The same method is
also recommended in FDA (2001) for assessment of PBE under the 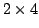 crossover design. However, we note that, asymptotically, FDA's PBE test has a
size smaller than the nominal level and thus has a low power to detect PBE.
In addition, the 2001 FDA guidance does not provide any statistical procedure
for PBE under commonly used
crossover design. However, we note that, asymptotically, FDA's PBE test has a
size smaller than the nominal level and thus has a low power to detect PBE.
In addition, the 2001 FDA guidance does not provide any statistical procedure
for PBE under commonly used 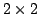 or
or 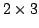 crossover designs.
In this paper, an asymptotically valid statistical test is derived for PBE
under the
crossover designs.
In this paper, an asymptotically valid statistical test is derived for PBE
under the 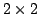 ,
, 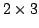 or
or 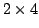 crossover design, using
the method of moments and linearization. A method of determining the sample
size required to achieve a desired power of the PBE test is also proposed.
Simulation results are provided to examine the performance of the proposed PBE
test and FDA's test. Finally, an example is presented for illustration.
crossover design, using
the method of moments and linearization. A method of determining the sample
size required to achieve a desired power of the PBE test is also proposed.
Simulation results are provided to examine the performance of the proposed PBE
test and FDA's test. Finally, an example is presented for illustration.
Key words and phrases:
Crossover design, drug prescribability, linearization, power, nominal level,
sample size.






crossover design and a statistical test procedure proposed by Hyslop, Hsuan and Holder (2000). The same method is also recommended in FDA (2001) for assessment of PBE under the
crossover design. However, we note that, asymptotically, FDA's PBE test has a size smaller than the nominal level and thus has a low power to detect PBE. In addition, the 2001 FDA guidance does not provide any statistical procedure for PBE under commonly used
or
crossover designs. In this paper, an asymptotically valid statistical test is derived for PBE under the
,
or
crossover design, using the method of moments and linearization. A method of determining the sample size required to achieve a desired power of the PBE test is also proposed. Simulation results are provided to examine the performance of the proposed PBE test and FDA's test. Finally, an example is presented for illustration.